Trapping under the Inversion
and Enhanced Chemistry
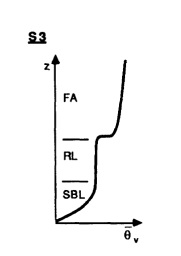 Fig. 1 [15] | Figure 1. shows a typical temperature profile of the atmosphere around 5 am local time. The very stable structure does not allow deep mixing like in the daytime convective boundary layer. Pollutants are distributed vertically depending on the altitude at which emission or chemical production occurs. Since the SBL and RL are essentially decoupled, pollutant profiles can vary. For example, vehicular emissions at the ground can become trapped at the surface due to the lack of mixing. Gases from car exhaust like carbon monoxide, nitrogen monoxide, and carbon dioxide can be a hazard to our health and the environment. The concentrations of these pollutants can increase throughout the night and disperse little in the horizontal and vertical. Pollutants emitted in the residual layer can be stored there. [2] |
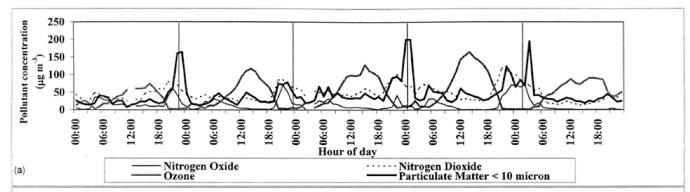
Salmond et al. dicussed turbulence and the structure of stable boundary layer in relation to air quality. A main point of their paper includes increasing the understanding between transport and diurnal concentration patterns. Observations from the Lower Fraser Valley (LFV) of British Columbia were taken during a 5 day period for various chemical species. Results showed a nocturnal peak in many pollutants including nitrogen oxide and PM10 . This was due to the trapping of surface emissions in the SBL and transport of chemicals from aloft. The increase in concentrations at night can be dangerous if over the National Ambient Air Quality Standards (NAAQS). [2]
Enhanced Chemistry
Another process happening withing a stable boundary layer is removal of ozone. Ozone is produced during daytime due to photochemistry (breakdown of molecules by sunlight), but at nighttime, it is removed by two processes:
1) Deposition onto vegetation and other surfaces. It is a permanent and irreversible process;
2) Chemical reaction, or titration with nitrogen oxide (NO). Emissions of NO may come from traffic and other near-surface combustion activities. Ozone reacts with NO to produce NO2 and oxygen molecules. However, when the sun rises in the morning, photochemistry helps convert NO2 and O2 back to ozone and NO, so this process is not permanent. [3]
These processes may cause the concentration of ozone to drop to zero at nighttime; however, producing another pollutant at the same time. According to the EPA website, NO2 is one of the six most common air pollutants that can be harmful to humans' lives. [11]
HNO3 goes through a similar process as ozone. Its concentration varies throughout the day, with the maximum during the daytime and minimum during nighttime. This process is enhanced even more in stable conditions. According to Dorsey et al. (2001), if under stable conditions a buildup of ozone occurs, it can result in formation of acidic gases and fine particle mass [4].
The study described below shows that under stable conditions that occur in the morning, concentrations of pollutants, such as CO and NOx were increased.
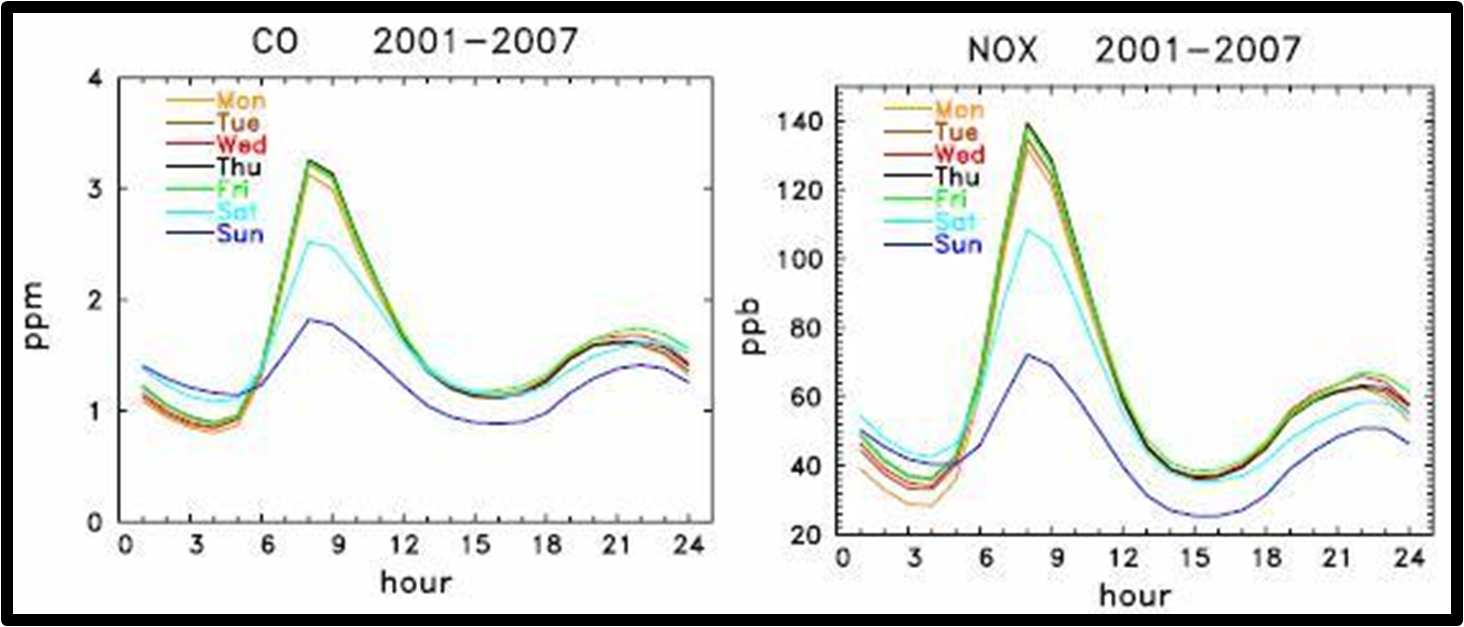
In a study by Stephens in 2008, weekly patterns of surface concentrations in Mexico City were analyzed. Lower concentrations appeared on Saturday and Sunday do to a phenomena called the 'weekend effect'. There are more emissions during workdays when traffic is increased. The two peaks represent morning and evening rush hour. This drastic difference in concentrations depends on the stability of the atmosphere. In the early morning hours, a stable layer is present before the daytime convective boundary layer forms. The pollutants are trapped under the nocturnal inversion. Before sunset, there is a large mixed layer allowing for more mixing and dispersion of pollutants leading to decreased concentrations [12].
Additional Information
 | A Darkness in Donora When smog killed 20 people in a Pennsylvania mill town in 1948 |